
February 25, 2022 from the Israeli Ministry of Health
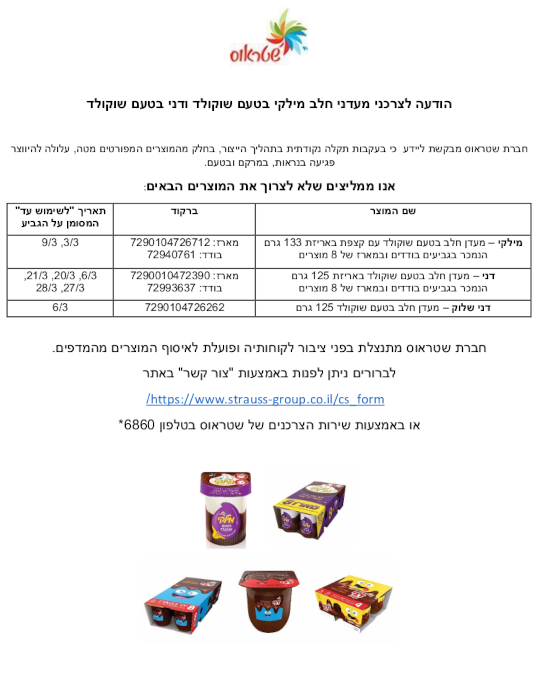
Notice to Consumers of Chocolate-Flavored Milky Danny and Danny Shlo : An isolated problem in production may have affected appearance, texture, and flavor


March 8, 2022 from the OU:
Wellsley Farms Blueberry Waffles; Chocolate Waffles, BJ's Wholesale, Westborough MA has a missing D - Dairy Designation:
The Orthodox Union certifies Wellsley Farms Blueberry Waffles and Chocolate Waffles as OU D - Dairy products. Some labels were printed with a plain OU mark, without the D - Dairy designation. Corrective actions are being implemented.
March 2, 2022 from the OU:
Nezka, Miami FL has an unauthorized OU:
The Orthodox Union does not certify Nezka products. Some Nezka labels bear an unauthorized OU mark. Corrective actions are being implemented.


February 25, 2022 from the OK in respose to a question from the a reader:
"Some of the Morningstar Incogmeato products, but not all of them are certified at this time – I believe the frozen product line is certified, while the refrigerated line is not currently certified."
Ed. note: Check each product for the kosher symbol.

March 2, 2022 from the OU:
Theo's, JVM Sales, Linden NJ has an unauthorized OU:
The Orthodox Union does not certify Theo's Feta Cheese. Some labels bear an unauthorized OU mark. If you see this product in the marketplace, please send details to kosherq@ou.org. Corrective actions are being implemented.
ANISAKIS WORMS IN FISH: The Halachic status of anisakis worms that are found in the flesh of many types of fish, is a matter of much dispute among the Poskim. Many American Kashrus certifications, including the Vaad Harabbonim / Council of Orthodox Rabbis of Greater Detroit, allow these fish. Consult your Rav for guidance. For those who wish to avoid this issue, a list that details which types of fish are affected is available on the COR website at COR Detroit Anisakis list
Ed. note: The following are articles explaining the kashrus issues of parasites in fish:
SOME LIQUOR COMPANIES ARE JEWISH OWNED and do not sell their chometz, and their products, including a number of varieties of American Whiskey, Canadian Whisky, Bourbon, Rye and Scotch, are therefore not permitted due to chometz she’ovar olov haPesach. An extensive list is available on the cRc [Chicago Rabbinical Council] website at https://www.crcweb.org/LiquorList.pdf . Following are a few examples of common liquors on that list (see details there):


July 12, 2021 and March 2, 2022 from the OU in respose to a question from the editor:
Pedialyte is labeled OUD, but in fact, its true status, at the present time, is DE (Dairy Equipment)..
Walgreens Electrolyte Solution is OU Pareve.
What does DE mean? A DE product does not contain actual dairy ingredients, but it is manufactured with heat on dairy equipment. DE items may be eaten after meat, but not with meat at the same time.
Many products qualify to be DE but are labeled OUD because OUD is a less confusing symbol for the kosher consumer. Also, to be a true DE product, the equipment must be properly cleaned of residue after dairy production, and that level of cleanliness is sometimes difficult to maintain and guarantee.
Please note that it is possible that the manufacturer will reformulate this product and add a true dairy ingredient. You will not be able to know this, since the OUD kosher symbol will remain the same. We recommend that you contact our office every few months to reconfirm the DE status of this product.
Walgreens Electrolyte Solution is OU Pareve.
The following kashrus information is from HaModia of June 29, 2011 and confirmed by the OU.
Pedialyte is certified as dairy, OU-D, because equipment had been used for processing of dairy without a proper kashering and does not contain any dairy ingredients.

February 28, 2022 from the FDA
The FDA, along with CDC and state and local partners are investigating consumer complaints and/or reports of infant illness related to products from Abbott Nutrition’s Sturgis, MI facility. All of the ill patients are reported to have consumed powdered infant formula produced from Abbott Nutrition’s Sturgis, MI facility. As of February 28, CDC has announced one additional illness of Cronobacter sakazakii with exposure to powdered infant formula produced at Abbott Nutrition’s Sturgis, MI facility. Cronobacter infection may have been a contributing cause of death for this patient. In total, this investigation includes four reports of Cronobacter sakazakii infections in infants (three from FDA complaints and one from a CDC case finding) and one complaint of a Salmonella Newport infection in an infant. All five (four Cronobacter infections and one Salmonella Newport infection) illnesses resulted in hospitalization and Cronobacter may have contributed to death in two patients. The most recent patient was reported to have consumed Abbott Nutrition’s Similac PM 60/40 product with the lot code 27032K800 prior to Cronobacter sakazakii infection. FDA and CDC informed the firm of these findings and on February 28, 2022, Abbott Nutrition voluntarily recalled Similac PM 60/40 powdered infant formula with the lot code 27032K800. This is a specialty formula for certain infants who would benefit from lowered mineral intake and was not included in the previous recall. At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.
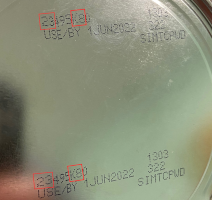
This particular lot of Similac PM 60/40 was distributed to the U.S. and Israel. If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices. ecommendation The FDA is advising consumers not to use recalled Similac, Alimentum, or EleCare powdered infant formulas. Recalled products can be identified by the 7 to 9 digit code and expiration date on the bottom of the package (see image below). Products are included in the recall if they have all three items below:
n addition to products described above, Abbott Nutrition has recalled Similac PM 60/40 with a lot code 27032K80 (can) / 27032K800 (case). At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.
Additional recall information for the initial recall is available on the FDA website. Parents can also enter their product lot code on the https://www.similacrecall.com/us/en/home.html">company’s website to check if it is part of the recall.
If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices.
f you get infant formula through WIC, do not throw the formula out. Instead, you should take it to the store for a refund and exchange or call the company at 1-800-986-8540 to help you. WIC recipients should be able to obtain a different brand of similar formula. Call your local WIC clinic for more guidance.




February 3, 2022 from the FDA and on February 23, 2022 at FDA:
Golden Medal Mushroom Inc. of Los Angeles, CA is recalling all cases of its 200g/7.05-ounce packages and 150g/5.25-ounce packages of Enoki Mushrooms (Product of China) because it has the potential to be contaminated with Listeria monocytogenes,
The recalled products were distributed to (Chicago IL/ Los Angeles CA/Dallas TX) through produce distributors or wholesalers to retail stores by 1/4/2022 and the affected lot number is 300511.
The Enoki comes in a 200g/7.05ounces, UPC: 6953150100684 and 150g/5.25ounces UPC: 6953150110157. Product is packaged in a vacuum sealed plastic package with upper transparent and lower portion has a black background. The product is “Product of China” and there is no English translation on label. There are no business name & address printed on packages.
Consumers who have purchased Enoki mushroom are urged to discard of or return them to the place of purchase for a full refund. Consumers with question may contact the company at (323) 720-9126, Monday- Friday 6:00AM to 1:00PM PST.
| The information posted is from secondary sources. We cannot take responsibility for the accuracy of the information. |
Copyright © kashrut.com. Permission is granted to reprint these alerts in hardcopy print media if kashrut.com is credited as the source of the information.
כל האומר דבר בשם אומרו, מביא גאלה לעולם אבות ו"ו
| Comments to webmaster@kashrut.com
© Copyright 2025 Scharf Associates |
|
|||||||||||