

February 28, 2022 from the FDA
The FDA, along with CDC and state and local partners are investigating consumer complaints and/or reports of infant illness related to products from Abbott Nutrition’s Sturgis, MI facility. All of the ill patients are reported to have consumed powdered infant formula produced from Abbott Nutrition’s Sturgis, MI facility. As of February 28, CDC has announced one additional illness of Cronobacter sakazakii with exposure to powdered infant formula produced at Abbott Nutrition’s Sturgis, MI facility. Cronobacter infection may have been a contributing cause of death for this patient. In total, this investigation includes four reports of Cronobacter sakazakii infections in infants (three from FDA complaints and one from a CDC case finding) and one complaint of a Salmonella Newport infection in an infant. All five (four Cronobacter infections and one Salmonella Newport infection) illnesses resulted in hospitalization and Cronobacter may have contributed to death in two patients. The most recent patient was reported to have consumed Abbott Nutrition’s Similac PM 60/40 product with the lot code 27032K800 prior to Cronobacter sakazakii infection. FDA and CDC informed the firm of these findings and on February 28, 2022, Abbott Nutrition voluntarily recalled Similac PM 60/40 powdered infant formula with the lot code 27032K800. This is a specialty formula for certain infants who would benefit from lowered mineral intake and was not included in the previous recall. At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.
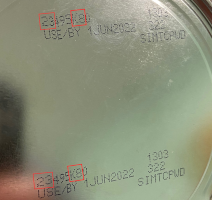
This particular lot of Similac PM 60/40 was distributed to the U.S. and Israel. If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices. ecommendation The FDA is advising consumers not to use recalled Similac, Alimentum, or EleCare powdered infant formulas. Recalled products can be identified by the 7 to 9 digit code and expiration date on the bottom of the package (see image below). Products are included in the recall if they have all three items below:
n addition to products described above, Abbott Nutrition has recalled Similac PM 60/40 with a lot code 27032K80 (can) / 27032K800 (case). At this time, Similac PM 60/40 with lot code 27032K80 (can) / 27032K800 (case) are the only type and lots of this specialty formula being recalled.
Additional recall information for the initial recall is available on the FDA website. Parents can also enter their product lot code on the https://www.similacrecall.com/us/en/home.html">company’s website to check if it is part of the recall.
If your regular formula is not available, contact your child’s healthcare provider for recommendations on changing feeding practices.
f you get infant formula through WIC, do not throw the formula out. Instead, you should take it to the store for a refund and exchange or call the company at 1-800-986-8540 to help you. WIC recipients should be able to obtain a different brand of similar formula. Call your local WIC clinic for more guidance.
| The information posted is from secondary sources. We cannot take responsibility for the accuracy of the information. |
Copyright © kashrut.com. Permission is granted to reprint these alerts in hardcopy print media if kashrut.com is credited as the source of the information.
כל האומר דבר בשם אומרו, מביא גאלה לעולם אבות ו"ו
| Comments to webmaster@kashrut.com
© Copyright 2025 Scharf Associates |
|
|||||||||||