
THIS SECTION IS FOR NEWS AND INTERESTING STORIES RELATED TO FOOD, NUTRITION AND FOOD PROCESSING. THEY ARE NOT NECESSARILY RELATED TO KOSHER BUT MAY BE OF INTEREST TO THE KOSHER CONSUMER, MANUFACTURER OR MASHGIACH.

February 24, 2026 from the CPSC :
The U.S. Consumer Product Safety Commission (CPSC) is warning consumers to immediately stop using Gourmia Pressure Cookers because they pose a risk of serious injury due to burn hazards. The lid on the pressure cooker can open while it is still pressurized, causing hot contents to spray out, resulting in severe second degree burn injuries to consumers. Most of these pressure cookers were sold at Best Buy.
In addition, the pressure cooker’s float valve, which is designed to raise when there is pressure in the product, and drop when there is no pressure, is located inside the handle, and is difficult to see. Consumers may not see that the float valve is still raised and may reasonably think it is safe to open the lid when the pressure cooker is under pressure.
The pressure cooker also has incorrect volume markings on the inner pot. This can cause consumers to overfill the pot and hot food and liquids to be ejected when the pressure cooker is vented using the quick release method or opened while its contents are pressurized.
The importer, the Steelstone Group, LLC doing business as Gourmia, of Brooklyn, N.Y., and the retailer, Best Buy Co., Inc. of Richfield, MN, which sold most of the products, have refused to agree to an acceptable recall to address this hazard.
CPSC has received five reports of incidents in which hot contents were expelled under pressure; in four cases, severe burn injuries were reported. There have been at least two lawsuits filed by consumers alleging burn injuries.
About 43,500 pressure cookers were sold between 2017 and 2020 at Best Buy, other retailers, and e-commerce platforms for between $50 and $80.
The pressure cookers are digital pressure cookers, with a cooking chamber capacity of six quarts, stainless steel and black plastic finishes, a pressure lid, a digital temperature and function display, and button controls. The model number is GPC625.
February 24, 2026 from Yeshiva World:
"srael’s Chief Rabbinate has issued a public warning over multiple imported food products found to carry misleading or unauthorized kosher certifications, raising concerns about kashrus and consumer transparency.
"n a special update, the Kashrut Fraud Division of the Chief Rabbinate of Israel detailed deficiencies involving dairy, fish, and meat products sold in major food chains.
"One case involves long-life whole milk produced in Belgium by Solarec and imported by Euro Dairies Europe (Gold Frost) Ltd. of Yavne. The cartons claimed supervision by Badatz Beit Yosef and approval from the Chief Rabbinate, presenting the product as chalav Yisrael.
"However, inspectors from the Rabbinate’s Import Department found that the milk was never authorized. As a result, officials ordered the product to be removed from shelves and returned to the importer.
"The Rabbinate also cited serious problems with “100% smoked cod liver in fish oil,” imported by G. Willifood International Ltd.. Although the packaging displayed kosher approval and supervision by the Orthodox Union, the Import Department had rejected approval requests for production batches beginning in January 2025.
"According to the Rabbinate, the rejection stemmed from missing documentation regarding parasite treatment and uncertainty over compliance with bishul Yisrael requirements.
"n the meat sector, inspectors identified misleading labeling on frozen beef shank imported from Argentina and marketed by Tnuva. An on-site inspection found that outer packaging described the meat as kosher-chalak, while the stamp on the meat itself indicated only standard kosher certification.
"The Rabbinate said the discrepancy could mislead consumers who rely on stricter kashrut standards.
"The Kosharot organization urged shoppers and supervisors to closely examine certification notices."
February 23, 2026 from Israel National News:
"The Knesset’s Health Committee has raised serious concerns over agricultural produce entering Israel from the Palestinian Authority. According to the latest data, nearly half of cucumbers and tomatoes tested contain banned pesticides linked to serious illnesses, including cancer and Parkinson’s disease.
"The committee, chaired by MK Limor Sohn Har-Melech, reported that roughly 15,000 tons of Palestinian agricultural produce cross into Israel each year. Between 2015 and 2022, 27%-40% of samples were found to exceed safety limits, with contamination levels steadily rising.
"Dr. Ziva Hamma of the Ministry of Health presented detailed findings: 50% of cucumber samples, 49% of tomato samples, and 66% of hot pepper samples were contaminated. Alarmingly, 14% of samples contained neurotoxic organic phosphorus, which can harm fetal and infant development and increase the risk of Parkinson’s. Another 13% contained a mix of more than five different pesticides in a single vegetable, posing risks to the liver, kidneys, and nervous system.
"Despite regulations requiring produce to be held until lab results are received, contaminated goods were often sold immediately. Amos Zuarets, Health Coordinator for the West Bank, admitted that the Civil Administration prioritized shelf life and the Palestinian economy over public health-an approach that changed only after the October 7 events. In eight months, all produce will be held until safe test results are confirmed.
"The Ministry of Health and Civil Administration plan several measures, including a computerized list of approved farmers, field inspections before produce enters Israel, increased sampling at crossings, and heavy fines for violators.
"Sohn Har-Melech concluded with a warning to the public: 'Nearly half of imported produce is contaminated with substances that threaten health, yet some prioritize profit over the safety of Israeli citizens. Check carefully where your fruits and vegetables come from and choose supervised Israeli produce.'"
February 19, 2026 from Matzav.com:
"Amid rising tensions with Iran and the ongoing month of Ramadan, concerns are mounting that several basic food items could quickly become scarce in Israeli supermarkets. According to a report in Maariv, the combination of heightened security concerns and a significant flow of food supplies into Gaza may lead to shortages of key staples in the coming weeks.
"Among the primary products at risk are fresh chicken and poultry cuts. Israel’s poultry industry relies heavily on Muslim workers — both Israeli citizens and Palestinians — particularly in slaughterhouses. Any security incident that restricts movement along the Jerusalem–Hebron corridor or at entrances to communities in Judea and Samaria can directly disrupt the supply chain, reducing the amount of fresh chicken reaching store shelves each morning.
"Eggs are also considered vulnerable. Israel has previously struggled to maintain sufficient production and imports during periods of heightened demand. Each year between January and April, emergency import quotas are typically opened to prevent sharp price increases. This year, with demand reportedly higher due to Ramadan and increased regional pressures, the risk of shortages is seen as more pronounced.
"Fresh fruits and vegetables present another area of concern. Unlike dry goods, these items cannot be frozen or stored for extended periods. The war has already exposed structural weaknesses in the agricultural sector, including a reduced number of foreign laborers, restrictions on Palestinian workers, and the halt of agricultural imports from several countries that previously supplied produce to Israel.
"Basic pantry staples such as flour, rice, oil, and sugar may also be affected. While Israel maintains what officials describe as a “living” reserve of wheat, the supply is not unlimited. The country remains highly dependent on grain and vegetable oil imports from Ukraine, other Black Sea nations, and South America. An escalation that disrupts maritime shipping routes — even if limited in scope — would not necessarily cause immediate shortages, but could result in a reduced variety of brands and gradual price increases within three to four weeks.
"Industry analysts caution that while panic buying is not currently evident, the combination of geopolitical tension, seasonal demand, and supply chain vulnerabilities creates a fragile situation for essential food products."
February 16, 20265 from VINnews:
"Following the introduction of new food products to the Israeli market, including ingredients produced using advanced technologies and containing milk protein not derived from animals, Israel’s Ministry of Health clarified that labeling a product as “parev” or “vegan” does not indicate the absence of allergens, including milk allergens.
"The Ministry of Health stated: 'Recently, a protein known as BLG (β-Lactoglobulin) was approved for use in food and is being added to products not made from animal sources. For individuals with a milk allergy, this is a milk allergen that may cause a severe, even life-threatening allergic reaction just like regular milk.'
"Products containing this ingredient may be labeled 'parev' or 'vegan,' but they are not suitable for individuals with a milk allergy and must include milk allergen labeling in the ingredients list. The Ministry of Health emphasized that the only reliable way to verify the presence of allergens is by reading the ingredient list on the product label.
"The Israeli Association of Allergy and Clinical Immunology responded: “The association warned the Ministry of Health several months ago about the risks of marketing food products labeled as ‘parev’ or ‘vegan’ while containing milk protein.”
"The association added: 'Patients with milk allergies are advised to avoid parve products due to the possibility of milk traces. Despite this, many rely on the "parev" label as an indication of safety. Therefore, the use of the term ‘parev’ may be misleading and dangerous. Moreover, such products may not only be consumed directly but also used in baking and cooking, including in kosher meat restaurants.'"
February 5, 2026 from the Nutraceuticals World:
"The U.S. Food and Drug Administration (FDA) announced that products without petroleum-based colors will be allowed to claim ‘no artificial colors.’ In the past, companies were generally only allowed to make the ‘no artificial colors’ claim if they had no added color whatsoever.
"The agency first announced this in a letter to the industry signaling their intent to exercise enforcement discretion on the matter. The move, officials say, will further incentivize companies that choose to use naturally-derived colors.
"The agency also approved beetroot red as a new color option, and approved a petition for the expanded use of spirulina extract. The administration has now approved six new food color options."
Ed. note: This change in pollicy is not good for consumers, since we will no longer know what colors have been added to foods.
Ed. note: Carmine and Coccineal extract are natural non-kosher insect-derived colors. They need be declared by their specific names, because of allergic reactions. Grape derived colors can be now added without informing the conssumer


February 12, 2026 from the CPSC :
The U.S. Consumer Product Safety Commission (CPSC) is warning consumers to immediately stop using PandaEar children’s portable hook-on chairs. The portable hook-on chairs violate the mandatory safety standard for portable hook-on chairs because the crotch restraint can be removed, posing a risk of serious injury or death from a fall.
CPSC issued a Notice of Violation to the seller, PandaEar of Lake Dallas, Texas. CPSC has requested that PandaEar recall the hook-on chairs and provide a remedy to consumers, but PandaEar has not agreed to an acceptable recall.
About 8,950 portable hook-on chairs were sold online at Amazon.com from February 2022 through November 2025 for about $25. The product was also sold on pandaear.com and by various third-party sellers and on other websites.
PandaEar portable hook-on chairs are used to seat young children at the table. The chairs have a black or gray metal frame covered with black or gray polyester and cotton material. There are two metal arms that anchor to a dining table and the child is suspended from the table. “PandaEar” and “Model BTC-51” can be found on the packaging. There is no labelling information on the product.
CPSC urges consumers to stop using the portable hook-on chairs immediately and dispose of them. Do not sell or give away these hazardous products.
Sold At: About 8,950 portable hook-on chairs were sold online at Amazon.com from February 2022 through November 2025 for about $25. The product was also sold on pandaear.com and by various third-party sellers and on other websites.
February 10, 2026 from the ch10.co.il
"Rabbi Hananel and Hodiya Cohen, a couple from Tiberias, have found themselves in recent months facing a huge lawsuit for 2.5 million shekels filed against them by the fruit and vegetable marketing giant - 'Yivoli Hakfar'.
"The reason for the lawsuit? A positive recommendation that was published about brands of leafy vegetable companies to consumers in the Mehadrin region, without including the company 'Yibul HaKfar' in the recommendations.
"According to a report today (Tuesday) by Shiloh Fried on the Ynet website, Rabbi Cohen has been recognized for many years as one of the experts on the subject of kashrut, and in particular on the subject of insect infestation in leafy vegetables. He is a kashrut consultant and is considered to have extensive and recognized professional knowledge. Because of this, religious councils, rabbis, and various kashrut bodies use his opinion to establish procedures, guidelines, and lists of product uses.
"In recent years, Rabbi Cohen, along with other supervisors specializing in the field, has expanded the scope of the tests they conducted on various leafy vegetable companies. The test results were summarized in a table that includes companies whose products were found to comply with the procedures of the Chief Rabbinate of Israel. The table was defined as a recommended list for Mehadrin kosher consumers.
"Lists of this type are not unusual in the world of kashrut. Similar lists have been published in the past by professional and official bodies, including the Chief Rabbinate, the 'Hikr' Institute, and the 'Koshrut' organization. The company was also not included in other published rankings.
"The table was used by rabbis and kashrut bodies for decision-making in businesses operating at the Mehadrin level, as well as by private consumers who wanted to ensure a high level of kashrut. ccording to Fried's report on Ynet, Cohen was surprised to discover that the company 'Yivoli Hakfar', which was not included in the list, filed a civil lawsuit against him "for damages caused to the plaintiff up to the date of filing the lawsuit only, as well as serious harm to the plaintiff's reputation and violation of the Commercial Torts Law.".
"The company also demands that the Jerusalem District Court "prohibit the defendants from publishing any ratings of companies regarding leafy vegetables in the context of kashrut under any trade name," as well as an injunction prohibiting them from presenting themselves to the general public as authorities in the field of kashrut and/or publishing information on the subject.
"Rabbi Hananel Cohen told Ynet: "When I received the lawsuit, I was shocked, it was a terrible feeling. Thank God I was able to act for the public in the most careful way possible, nothing for my own benefit.".
"Attorney Meir Ehrenfeld told Ynet on behalf of Rabbi Hananel Cohen: "The attempt by 'Village Crops' to use the court as a tool for commercial revenge is transparent and desperate. The lawsuit for 2.5 million NIS is a classic gag order, intended to punish Rabbi Hananel Cohen for his professional integrity. It would have been better for 'Village Crops' to invest in public health and cleaning its products of insects, rather than conducting legal and propaganda campaigns that are empty of content and lacking any support.".
"Attorney Shahar Botton, the representative of the Yavuli Hakfar company, said in response to Ynet: "There is no substance in the claims made to our firm, the reality is completely the opposite. Mr. Hananel Cohen is not the weak side in this matter and this is not a claim for silence, on the contrary, Mr. Hananel Cohen is the strong side."



February 5, 2026 from the CPSC :
Lancaster Table & Seating Powder-Coated Aluminum Outdoor Chairs and Barstools have been recalled because the legs of the chairs and barstools can bend or break while the chair is in use, posing a fall hazard.
Consumer Contact: Clark Associates toll-free at 800-285-8172 from 9 a.m. to 5 p.m. ET Monday through Friday, by email at pcachairsandbarstools@lancastertableandseating.com or online at www.lancastertableandseating.com/recalls/powder-coated-aluminum-chairs or www.lancastertableandseating.com/ and click on “Product Recalls” at the bottom of the page for more information.
Description: This recall involves three models of Lancaster Table & Seating Powder-Coated Aluminum Outdoor Chairs and Barstools in arm chair, side chair (no arms) and barstool configurations sold under 180 item numbers. The chairs and barstools were sold in blue, brown, dark gray, light gray/silver, red, white, aqua, black, green, sangria/burgundy, yellow and orange. The chairs were sold with and without accompanying outdoor tables, which are not subject to the recall. The item numbers can be found on order confirmation emails, shipping confirmation emails and invoices. A full list of affected item numbers and examples of colors are available at www.webstaurantstore.com/uploads/Clark_Associates/2026/1/lts-recall-list-of-affected-products-1.pdf.
Remedy: Consumers should immediately stop using the recalled chairs and barstools and contact Clark Associates for a full refund to the original method of purchase or a store credit. Consumers should register their product online at www.lancastertableandseating.com/recalls/powder-coated-aluminum-chairs, mark the product with the word “Recalled” in paint or thick permanent marker in a visible location and dispose of the product in accordance with local and state laws.
Incidents/Injuries: The firm has received 36 reports of broken or bent chair legs, resulting in four falls and three injuries.
Sold At: Clark Pro and Clark National Accounts nationwide and online at webstaurantstore.com, therestaurantstore.com and quicksupply.com from April 2020 through October 2025 for between $55 and $133.
Sold At: Clark Pro and Clark National Accounts nationwide and online at webstaurantstore.com, therestaurantstore.com and quicksupply.com from April 2020 through October 2025 for between $55 and $133.

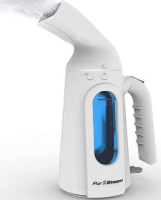

February 5, 2026 from the CPSC :
PurSteam Mighty Lil Steamers and PurSteam Elite Travel Steamers have been recalled because the steamers can expel hot water from the steam nozzle during use, posing a serious burn hazard to consumers.
Units: About 75,400 PurSteam Elite Travel Steamers and about 119,000 PurSteam Mighty Lil Steamers
Consumer Contact: Aterian toll free at 833-910-6095 from 9 a.m. to 5 p.m. ET Monday through Friday, by email at recall@pursteam.com, or online at recall.pursteam.com or at https://pursteam.com/ and click on “Product Recall” at the top of the page for more information.
ncidents/Injuries: Between December 2020 and 2024, there were 80 reports of hot water being expelled from the PurSteam Elite Travel Steamer model PS-510, including 14 reports of burn injuries. Between December 2020 and 2024, there were 392 reports of hot water being expelled from the PurSteam Mighty Lil Steamer model PS-550, including 40 reports of burn injuries, two of which were reported as second-degree burns. There are additional reports of hot water being expelled from both steamer models, including reports of burn injuries, from before Aterian’s acquisition of PurSteam in December 2020.
Description: This recall involves PurSteam Elite Travel Steamers and PurSteam Mighty Lil Steamers purchased after December 2020 or with one of the following date codes: 2310, 2308, 2305, 2304, 2303, 2212, 2211, 2210, 2203, 2112, 2111, 2110, 0221, 1019, and 4619. The PurSteam Elite Travel Steamer is white with a triangular-shaped opening for the steam vents. The “PurSteam” logo is printed below a wave-shaped water reservoir window, and “Model: PS-510” is printed on a label on the bottom of the product. The PurSteam Mighty Lil Steamer is white with an oblong-shaped opening for the steam vents. The “PurSteam” logo is printed below the water reservoir window, and “Model: PS 550” is printed on a label on the bottom of the product. On both steamer models, the manufacture date (date code) is located on a label on the bottom of the product in YYMM format.
Sold Online At: Pursteam.com, Amazon.com and Walmart.com from December 2020 through April 2025 for between about $15 and $35 for the PurSteam Elite Travel Steamer model PS-510, and from December 2020 through January 2024 for between about $11 and $30 for the PurSteam Mighty Lil Steamer model PS-550.
| The information posted is from secondary sources. We cannot take responsibility for the accuracy of the information. |
| Comments to webmaster@kashrut.com
© Copyright 2026 Scharf Associates |
|
|||||||||||